Sim4Web
Simulators for the Web (Sim4Web) is the platform that hosts our online simulations in a virtual laboratory held in “the cloud”
Experiment 6: Beer-Lambert Law and Determination of the Level of Aspirin in a Blood Sample & Tablet
Background

Aspirin (acetylsalicylate) [the structure on the left] is used to treat a number of conditions such as pain, inflammatory diseases including rheumatoid arthritis and to help prevent heart attacks and strokes. Since aspirin absorbs UV radiation between 200-340 nm it is generally more convenient for spectroscopic analyses, to form an intensely coloured 1:1 complex with iron (III) which shifts the wavelength of maximum absorption of the resulting complex into the visible range of the electromagnetic spectrum. The level of salicylate in blood plasma can be detected by several analytical techniques including UV-Vis absorption spectroscopy.
The Task
A lady suffering with severe arthritis was rushed into hospital with a suspected aspirin overdose. Your task is to detect the level of aspirin in a blood sample taken from the patient, by UV-Vis absorption spectroscopy, so that aspirin overdose can be confirmed and appropriate treatment administered. Also, you have been given an aspirin tablet taken from the scene of the suspected overdose. From UV-Vis spectroscopic analysis of the tablet, detect the level of aspirin present and confirm the dosage of the tablet.
Experiment 6, Determination of the Level of Aspirin in a Blood Sample & Tablet
The Beer-Lambert Law is used in the first part of the experiment which relates the absorbance to the concentration of the sample. Students will make up a series of solutions of acetylsalicylic in water using the virtual flask component of the wet bench module:
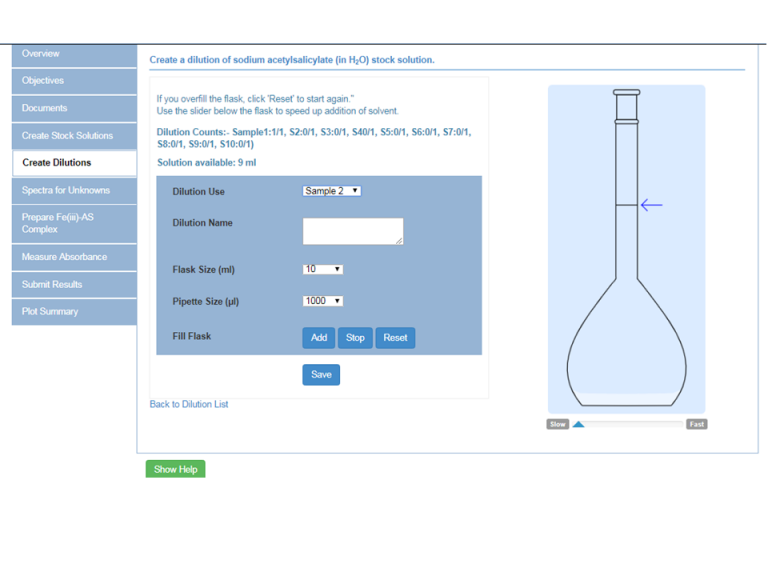
The virtual wet bench where the sodium acetylsalicylate and iron (iii) chloride complex is prepared:

The complex is prepared by adding iron (iii) chloride to the virtual sodium acetylsalicylate solutions.
The absorbance spectra of the virtual solutions will be recorded using the spectrometer simulator:

A Beer-Lambert plot will be constructed in order to derive the molar extinction coefficient for the sodium acetylsalicylate and iron (iii) chloride complex.
In part 2 of the experiment, students have been allocated a blood plasma sample and must determine the level of salicylate present using the information derived in part 1.
In part 3, students must determine the level of aspirin present in a tablet recovered from the scene of the suspected overdose using the information derived in part 1.
Any questions? Please contact our experts who will be happy to help.
We cover a variety of topics: Everything from studying the kinetics of the crystal violet/sodium hydroxide reaction to determining the level of aspirin in a blood sample. Take a look at our product page.
