Sim4Web
Simulators for the Web (Sim4Web) is the platform that hosts our online simulations in a virtual laboratory held in “the cloud”
Experiment 5, Beer-Lambert Law and the Temperature Dependence of the Crystal Violet-Sodium Hydroxide Reaction
Background

Crystal Violet, CV, (structure shown on the left) is an intensely coloured dye. Reaction with NaOH results in a colourless product. The absorbance of a reaction mixture containing CV and NaOH will be proportional to the concentration of unreacted dye still present in solution. The reaction can therefore be monitored and the kinetics studied by measuring the absorbance of the mixture as a function of time.
Arrhenius Behaviour
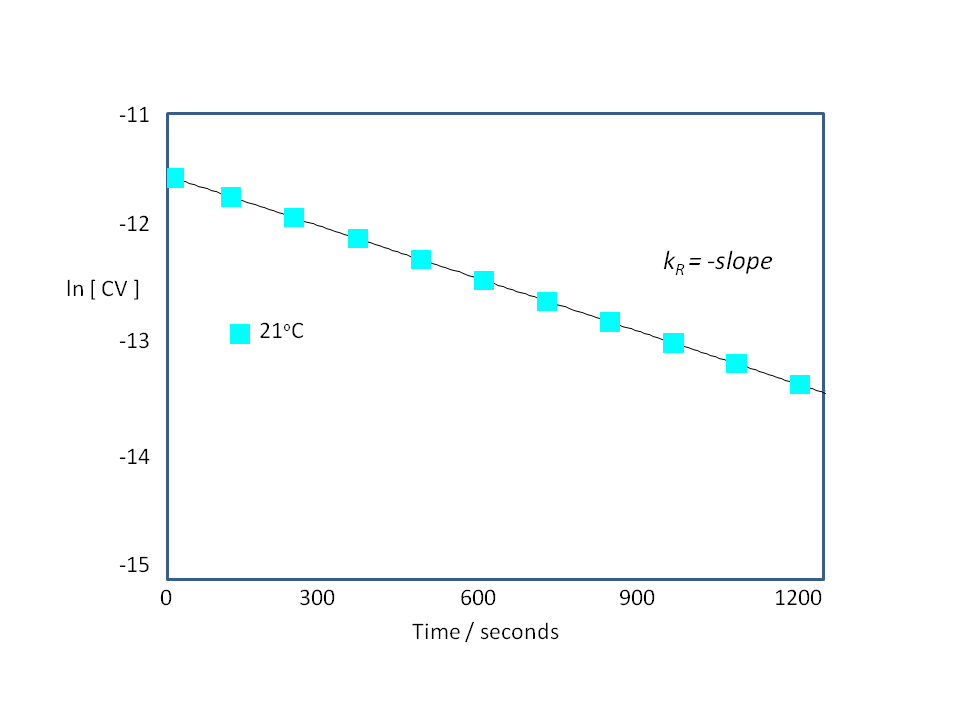
Since the CV/NaOH reaction follows first order kinetics under the conditions adopted in this experiment, then
ln [CV]t = ln [CV]o – kRt
A plot of, ln [CV] vs. time will be linear and kRt can be derived from the slope of the plot.
By using the Beer-Lambert law, if the molar extinction coefficient
for CV is known, the concentration of CV can subsequently be
derived as a function of time and kR determined.
The rate of many chemical reactions increase exponentially with temperature and can be described by the Arrhenius equation:
kR = A exp-Ea/RT
where A is the pre-exponential factor, Ea the activation energy and R is the gas constant.
The CV/NaOH reaction will be performed at five different temperatures. kR will be derived at each temperature.
Does the CV/NaOH reaction show Arrhenius behaviour?
Experiment 5, the Temperature Dependence of the CV/NaOH Reaction
The Beer-Lambert Law is used in the first part of the experiment which relates the absorbance to the concentration of the sample. Students will make up a series of solutions of CV in water using the virtual flask.

The absorbance spectra of the virtual solutions will be recorded using the spectrometer simulator:

A Beer-Lambert plot will be constructed in order to derive the molar extinction coefficient for CV. This information can then be used to determine the concentration of CV remaining in the reaction as a function of time in the second part of the experiment.
The CV/NaOH reaction will be performed in the virtual laboratory at five different temperatures in part two of the experiment.
Apparatus used in this experiment: Graduated flasks and various pipettes; timer; water bath; heater; thermometer; 1cm path length sample cell; pipette to release 9 cm3 of CV; pipette to release 1 cm 3 of NaOH to start the reaction; absorption spectrometer simulator.
The screen captures below show the experiment being performed at 21oC:
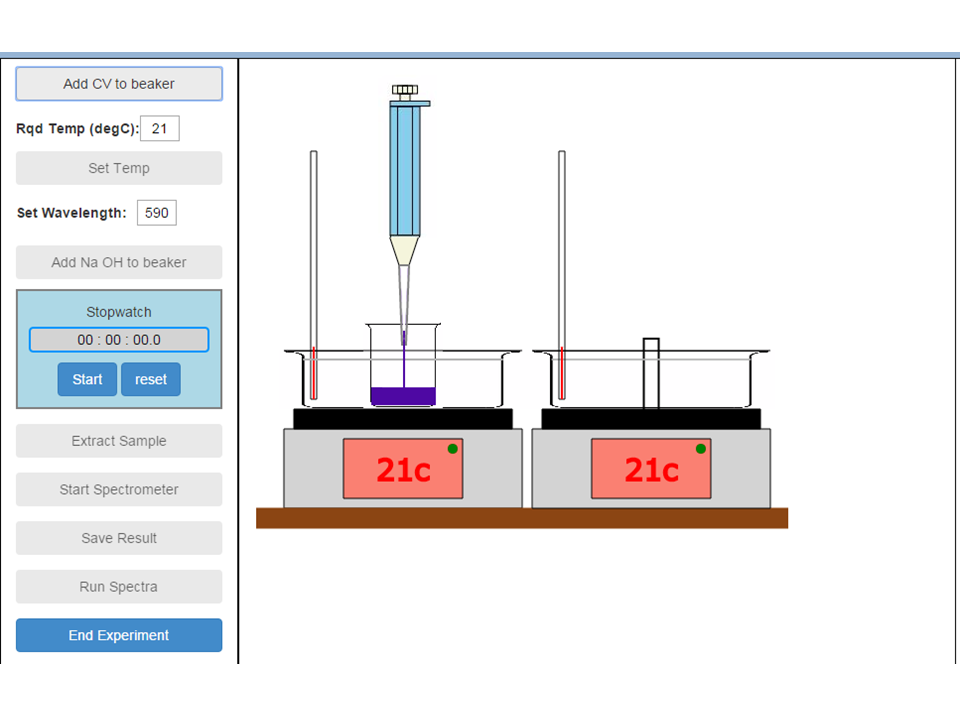
A sample of the CV/NaOH reaction mixture is extracted from the reaction and transferred to a cuvette. The cuvette is then loaded into the spectrometer and absorbance readings taken as a function of time:
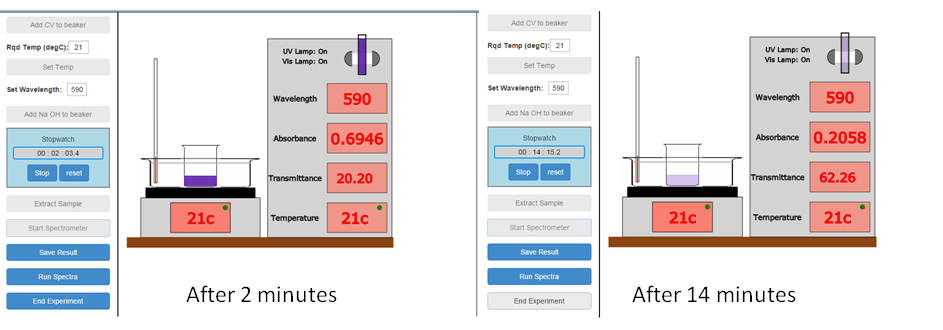
Sample Documentation, Look Inside:
Any questions? Please contact our experts who will be happy to help.
We cover a variety of topics: Everything from studying the kinetics of the crystal violet/sodium hydroxide reaction to determining the level of aspirin in a blood sample- we have it all! Take a look at our product page.

